Every physical substance exists in three states: solid, liquid, and vapor. A specific matter can only exist in one condition at a given temperature and pressure. The change in state corresponds to the change in heat content (either the matter itself or the surroundings). In general, heat must be supplied to a solid matter to convert it to liquid or a liquid matter to vapor. In the opposite circumstance, though, heat should be evacuated from the substance.
Even if this is true, no substance can exist in its basic physical state indefinitely. This is due to exogenous factors at work.
Evaporation and boiling is a topic under basic science and technology scheme of work. You will find a deeper explanation of the subject matter in JAMB syllabus for Physics.
Now let’s take a cursory look into the definitions and striking differences between boiling and evaporation. At the end of this post you should be able to define boiling and evaporation as well as distinguish between both processes.
What is boiling?
When a liquid is heated to the boiling point, it evaporates quickly. For example, when water is heated to 100 ° C on the sea’s surface, it begins to boil and transforms into steam. The microorganisms present are killed and softened by boiling water. It is also utilized in numerous cooking processes. The vapor pressure of the liquid can overcome air pressure during the boiling process. That is why bubbles can form and rise in boiling water.
What exactly is boiling point?
This is the temperature at which the liquid’s vapor pressure equals the ambient pressure and the liquid turns to vapor. A substance’s boiling point is affected by the surrounding pressure. Do you know that Liquids with high pressure have a higher boiling point than liquids with low pressure. Water boils at 100 ° C at sea level, however, in the highlands where atmospheric pressure is low, it boils at temperatures lower than 100 ° C.
2 Factors influencing boiling point
The boiling point is affected by the following factors:
1. The atmospheric pressure
The boiling point of a substance varies with its surroundings. The boiling point rises as the air pressure rises because more energy is required to break the connections between the particles. As a result, the high pressure raises the boiling point whereas low pressure decreases it.
2. Impurities
A compound’s boiling point is utilized as a reference for its pure state. Pure water, for example, boils at 100 ° C, whereas water containing other compounds and dirty water boil at a higher temperature. As a result, contaminants in a pure substance raise the boiling point.
What is Evaporation?
Fill a beaker halfway with water. Then, return this mug to the flame to continue heating. After heating for a bit, you will notice the water starting to boil and turning into steam. This is referred to as evaporation. Did you ever notice how, if a glass of water falls to the floor and no one washes it away, it eventually dries? Wet garments will eventually dry. Accomplish you understand how to do it? As we know, particles of matter are continually moving and never rest, hence they contain various amounts of kinetic energy at different temperatures.
Even in liquids, a tiny portion of particles with high kinetic energy on the surface can be transformed into vapors away from the gravitational pull of other particles. Evaporation is the process of turning a liquid into vapor at any temperature below its boiling point.
2 Factors Influencing Evaporation
Evaporation is an example of a surface phenomenon. Evaporation can be influenced by the following factors:
1. Temperature
Temperature rise is directly proportional to the rate of evaporation. As the temperature rises, more particles receive enough kinetic energy to convert to a vapor state. Wet clothes, for example, dry quickly in the sun.
2. Humidity
The quantity of water vapor in the air is referred to as humidity. At a particular temperature, air can only hold a finite amount of water vapor. The rate of evaporation slows when the amount of water in the air is already high or at maximum. When the air is dry, fewer water molecules return to the liquid, causing the water to evaporate faster. When the air contains a high concentration of water molecules (i.e., when it is humid), such as on a rainy day, evaporation is slower because more water molecules return to the liquid.
Try the following Experiments:
Step 1 – Fill three similar jars with an equal amount of water.
Step 2 – One jar should be left uncovered, another with aluminum foil, and the last with a tightly fitted lid.
Step 3 – What would you anticipate to observe after a few days to a week based on what we’ve learned?
Step 4 – The conclusion would be, the less evaporation there is, the tighter the lid.
Why is this so? People may argue that with a jar top on, the water molecules have nowhere to go. “Yes,” you could argue, “but can’t water molecules still escape into the air above the water in the jar?” They can, but it will result in a considerable increase in the number of water molecules in the jar’s air (higher humidity) and a significant increase in the number of water molecules returning to the liquid. It quickly reaches the point where the number of water molecules returning to the liquid equals the number of water molecules leaving the liquid, resulting in no net change in the quantity of water (i.e., no evaporation).
That is a far more intricate explanation than the lid merely keeping the water in. You probably won’t need to go into that much detail with your pupils, but knowing the process well will give you a lot more confidence in your material understanding.
3. Wind Speed
When you step out of an outdoor swimming pool while the wind is blowing, you will feel colder because the wind causes the water to evaporate faster from your skin, taking heat energy away from your skin faster and leaving your skin colder. This phenomenon is also responsible for the “wind chill factor” mentioned in weather reports on frigid winter days. Also, most times, you are not in a swimming pool, yet your skin is usually moist. The wind causes moisture to evaporate more quickly, removing more heat from your skin. That is why it is critical to stay warm and dry on chilly, windy days. As wind speed increases, water vapor particles travel away from the wind, reducing the amount of water vapor in the surrounding area. Both evaporation and boiling entail the conversion of a liquid to a gas, although they differ in several ways.
4. Surface area
Increasing surface area provides more surface area for water to evaporate. So, instead of being folded or bunched up, a wet towel will dry faster if it is spread out.
6 Differences Between Evaporation and Boiling
1. Speed
Evaporation is a slower process than boiling. Consider this: Set a tall glass of water on a shelf in the weeks leading up to a lecture on the water cycle (including evaporation and condensation), and have people measure the height of the water every few days and keep a table indicating the changing level. If the people preferably students don’t already know what’s going to happen, don’t tell them; instead, let them find it for themselves. They can also plot the water’s height over time. When you are ready to discuss evaporation, ask, “What happened to the water in the glass?”(This experiment might also be conducted with students reading the amount of water remaining in a graduated cylinder, but you may want to have students learn to measure using a ruler.) In contrast to the days and weeks it takes for water to evaporate, you may boil water on a hot plate and observe the water level drop in minutes.
2. The temperature required
Any temperature above freezing causes a liquid to evaporate. The water in the preceding example was at room temperature and slowly evaporated. If the temperature had been higher, the water would have evaporated faster. Boiling, on the other hand, occurs only when the liquid reaches a specified temperature, known as the boiling point.
3. Bubbles
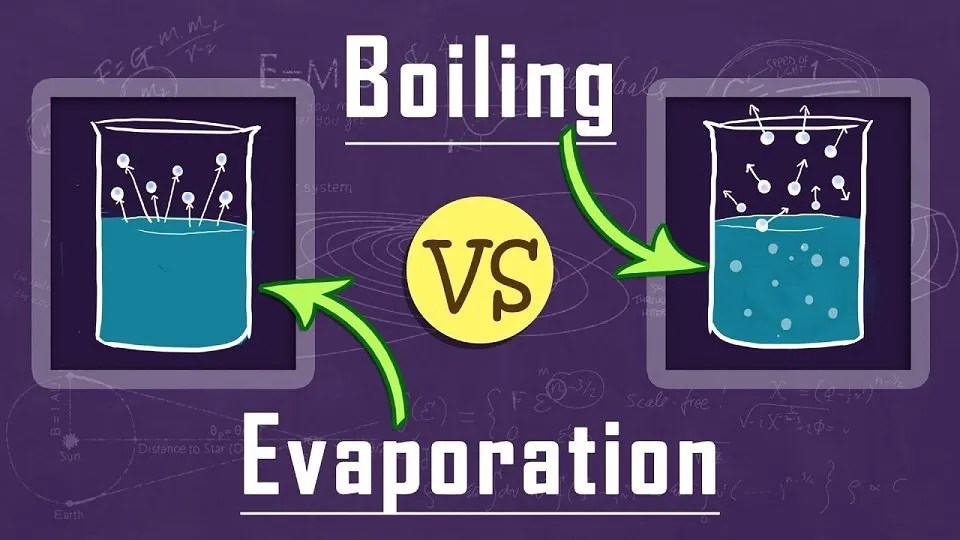
Evaporation does not result in the creation of bubbles. Individual molecules leave the liquid and become part of the air when it evaporates. Condensing processes are those that convert a gas to a liquid. When the water in your glass evaporated, molecules must have moved from the water to the air more frequently than molecules from the air to the water. As a result, water molecules were transferred from the liquid in the glass to the air. This process does not include any bubbles. When you boil water, the liquid changes to gas so quickly that bubbles of water vapor develop.
For students, consider the following think-pair-share question: Is there anything in the bubbles formed when boiling water? If that’s the case, what are the bubbles made of? Some kids may mistake those for air bubbles. But now you realize they are not air bubbles, but water vapor bubbles. Water that has converted from a liquid to a gas. Is there a simple way to show that the bubbles contain water molecules? Yes! Warm up a big pot of water. Place a lid on it for about 20 seconds, then remove the lid and inspect the inside. It’s covered in water droplets from the condensed bubbles on the cooler lid.
4. The location of the transition to a gas
You have probably seen that when you heat water to bring it to a boil, bubbles emerge initially on the bottom of the pot. This is because, initially, the bottom of the pot is the only location where it’s hot enough to heat the water to the boiling point and transform it into a gas. When you have a rolling boil, the entire pot of water is at the boiling point (100°C), and bubbles emerge throughout the majority of the water. Evaporation, on the other hand, happens solely on the water’s surface.
5. Energy source
Boiling typically necessitates the use of an external source of energy, such as the burner beneath the pot of water in which you’re boiling your eggs. Evaporation, on the other hand, utilizes the energy that is already present in the liquid. A puddle of water has some heat energy, which is normally derived from the surrounding environment. Some molecules move swiftly enough to escape and evaporate into the air because of the heat in the water. Evaporation requires no additional energy, and the water does not have to reach the boiling point to evaporate. Water, as we have seen, evaporates at normal temperature. Thus, it can be concluded that evaporation, but not boiling, is a natural process. Your pool of water or the water on your freshly cleaned hair will evaporate without your intervention. Just wait for it to dry. However, boiling does not occur spontaneously. To cause the liquid to boil, we must actively heat it.
6. Liquid temperature change
When water boils, its temperature remains constant at 100°C. A rolling boil does not raise the temperature of water over a medium boil. Your eggs will cook at the same rate in either case. Water evaporation, on the other hand, cools the water, as well as any surface that the water evaporates off. That is why you feel cold after getting out of the shower. Water molecules evaporating transmit heat away from your skin. On a hot summer day, this is also why you sweat. Greater moisture on your skin causes more evaporation, which cools it. So, instead of wiping the sweat from your brow, allow it to drain and you’ll feel much cooler!
Differences between Evaporation and Boiling are crucial to understanding because they are frequently confused. However, it is critical to understand the key distinctions and similarities between evaporation and boiling. Evaporation is the process through which liquid turns into vapor. To understand Evaporation, consider this: when water evaporates from the soil due to the heating action of the sun, this is referred to as Evaporation. Boiling, on the other hand, refers to the process of raising the temperature of a liquid to a level higher than the boiling point of the substance. The primary distinction between Evaporation and Boiling is that Evaporation occurs only on the liquid’s surface, whereas Boiling occurs across the entire bulk of the liquid. This is classified as the primary distinction between Evaporation and Boiling. Also, evaporation is slower, occurs exclusively at the liquid’s surface, does not generate bubbles, and causes cooling. Boiling is rapid, can occur throughout the liquid, produces a large number of bubbles, and has no cooling effect.